Design, synthesis and conformational analyses of bifacial benzamide based foldamers†
Abstract
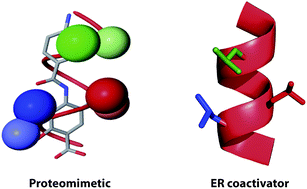
The design, synthesis and conformational analyses of novel backbones represents a key focus of research that underpins efforts to exploit foldamers (i) in a biological setting e.g. as inhibitors of protein–protein interactions (PPIs) and (ii) for the purposes of constructing functional architectures that adopt defined tertiary and quaternary folds. The current manuscript addresses a need to develop aromatic oligoamide backbones that are regioisomeric in terms of backbone connectivity and/or functionalized on more than one face. We describe the design, synthesis and comparative conformational analyses of foldamers derived from 2-, 3- and 2,5-O-alkylated derivatives of para-aminobenzoic acid, and, derived from 2-,3- and 2,5-O-alkylated derivatives of 1,4-diaminobenzene/terephthalic acid monomers. Analysis of the accessible conformational space for these oligomers indicates that despite different connectivity they can adopt conformations that position side chains in a manner that mimic the i, i + 3, i + 4 of an α-helix.

 Please wait while we load your content...
Please wait while we load your content...