Poly(ethylene oxide)-based block copolymers with very high molecular weights for biomimetic calcium phosphate mineralization†
Abstract
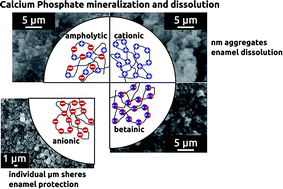
The present article is among the first reports on the effects of poly(ampholyte)s and poly(betaine)s on the biomimetic formation of calcium phosphate. We have synthesized a series of di- and triblock copolymers based on a non-ionic poly(ethylene oxide) block and several charged methacrylate monomers, 2-(trimethylammonium)ethyl methacrylate chloride, 2-((3-cyanopropyl)-dimethylammonium)ethyl methacrylate chloride, 3-sulfopropyl methacrylate potassium salt, and [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide. The resulting copolymers are either positively charged, ampholytic, or betaine block copolymers. All the polymers have very high molecular weights of over 106 g mol−1. All polymers are water-soluble and show a strong effect on the precipitation and dissolution of calcium phosphate. The strongest effects are observed with triblock copolymers based on a large poly(ethylene oxide) middle block (nominal Mn = 100 000 g mol−1). Surprisingly, the data show that there is a need for positive charges in the polymers to exert tight control over mineralization and dissolution, but that the exact position of the charge in the polymer is of minor importance for both calcium phosphate precipitation and dissolution.

 Please wait while we load your content...
Please wait while we load your content...