Study of the effect of molecular structure and alkyl groups bound with tin(iv) on their cytotoxicity of organotin(iv) 2-phenyl-4-selenazole carboxylates†
Abstract
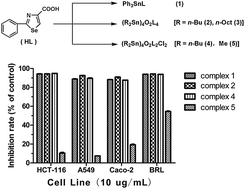
Five organotin(IV) compounds Ph3SnL (1), (R2Sn)4O2L4 [R = n-Bu (2), n-Oct (3)], (R2Sn)4O2L2Cl2 [R = n-Bu (4), Me (5)], have been synthesized from the reactions of 2-phenyl-4-selenazole carboxylic acid (HL) with the corresponding organotin(IV) oxide or chlorides. These compounds have been characterized by elemental analysis, IR, 1H, 13C and 119Sn NMR spectroscopy, and single crystal X-ray diffraction analysis. Structural studies reveal that compound 1 exhibits a mononuclear four-coordinated tetrahedral geometry. Differently, the crystal structures of compounds 2–5 reveal the formation of the tetranuclear species containing a planar Sn4O4 core. All compounds were screened for their in vitro cytotoxic activities toward three cancer cell lines (Caco-2, A549, and HCT-116) and one normal rat hepatocyte cell line (BRL). The results indicate that both di-n-butyltin(IV) and triphenyltin(IV) derivatives not only show excellent cytotoxic activities on cisplatin-sensitive lung cancer cell line A549 and but also exhibit good cytotoxicity against cisplatin-insensitive colon cancer cell lines HCT-116 and Caco-2. Whereas, dimethyltin(IV) and di-n-octyltin(IV) complexes exhibit lower or no cytotoxic activity. The structure–activity relationship of the cytotoxicity of the title complexes has also been discussed.

 Please wait while we load your content...
Please wait while we load your content...