Design, synthesis, anticonvulsant and analgesic studies of new pyrazole analogues: a Knoevenagel reaction approach†
Abstract
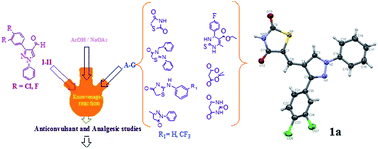
The present work involves the design and synthesis of a number of new compounds starting from 3-(3,4-dihalophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde. The compounds were synthesized by adopting the Knoevenagel condensation reaction to meet the structural prerequisite required for anticonvulsant and analgesic activities. The reaction of 3-(3,4-dihalophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde with substituted thiazolidine, pyrazolone, thiazolo[3,2-a]pyrimidine, Meldrum's acid and barbituric acid yielded a variety of heterocycles bearing the pyrazole moiety. The newly synthesized compounds were characterized by elemental and spectroscopic analysis; in addition, the structure of compound 1a has been elucidated by single crystal X-ray diffraction technique. The synthesized molecules were evaluated for their in vivo anticonvulsant activity using maximal electroshock seizure (MES) test, while their analgesic activity was investigated by tail flick method. Further, rotarod toxicity method was used to study the toxicity profile of the selected compounds. Among the synthesized compounds, 1a, 4a and 7a possessed potent anticonvulsant activity and 1b, 5a, 5b, 7a and 7b showed the highest analgesic activity without displaying any toxicity. Efforts were also made to establish structure–activity relationships among the tested compounds.

 Please wait while we load your content...
Please wait while we load your content...