Silyl-assisted 1,2-cis-α-glucosylation for the synthesis of a triglucoside moiety in high-mannose-type oligosaccharides†
Abstract
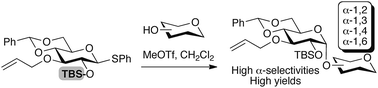
A highly stereoselective 1,2-cis-α-glucosylation reaction was developed, in which the use of a strongly electron-donating tert-butyldimethylsilyl protecting group on the C-2 hydroxy group of a glycosyl donor enhanced the α-favoured transition state, and thus resulted in high yield and α-selectivity. Synthetic utility of this α-glucosylation was demonstrated by the generation of a triglucoside moiety in high-mannose-type oligosaccharides.

 Please wait while we load your content...
Please wait while we load your content...