Mechanism of water separation from a gaseous mixture via nanoporous graphene using molecular dynamics simulation
Abstract
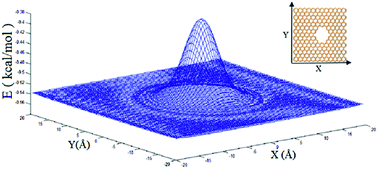
We present an investigation on the permeability and selectivity of graphene sheets with designed sub nanometer pores using molecular dynamics simulations. We show that nanoporous graphene can be used to separate water from a gaseous mixture of nitrogen, oxygen and water. The obtained results show that the separation is performed based on the absorption of water molecules on the nanoporous graphene surface. Pore size plays an important role in the possible formation of water clusters on the nanoporous graphene surface. In graphene sheets with large pores, the space between the neighboring graphene becomes smaller and water molecules form a cluster on the graphene surface quickly. Because the size of the water clusters is larger than that of the nanopore, they cannot pass through it, whereas nitrogen and oxygen molecules are able to pass through the nanoporous graphene. In this study, we propose a mechanism for the formation of water clusters on the nanoporous graphene surface. To illustrate the different steps of the proposed mechanism, several investigations were performed such as calculation of the distribution and accumulation of water molecules on the graphene surface, angle between the dipolar moment vector of water molecules and the graphene surface and formation and movement of multi-layer clusters. Moreover, a specific method to pass N2 and O2 molecules through nanoporous graphene is presented.

 Please wait while we load your content...
Please wait while we load your content...