Poly(ω-bromoalkylnorbornenes-co-norbornene) by ROMP-hydrogenation: a robust support amenable to post-polymerization functionalization†
Abstract
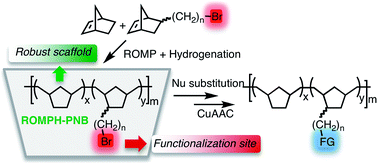
The ring opening metathesis copolymerization of norbornene and ω-bromoalkylnorbornenes NB–(CH2)nBr (n = 1, 4) by Grubbs' 2nd generation catalyst, followed by hydrogenation, gives insoluble saturated polynorbornenes (ROMPH-PNBs) that have pendant ω-bromoalkyl chains (4a, b). These materials can be functionalized by nucleophilic substitution of bromide to give a variety of substituted polymers (ROMPH-PNB–(CH2)nNu where Nu = CN, SPh, OOCMe, N3, SnR3. The stannylated polymers were tested in a Pd-catalyzed reaction, the Stille coupling. The azido polynorbornenes ROMPH-PNB–(CH2)nN3 easily undergo the click 1,3-dipolar cycloaddition with alkynes, which could be a useful strategy to anchor other functionalities of interest. The aliphatic nature of the ROMPH-PNB–(CH2)nBr backbone makes robust supports and the presence of the bromo substituent imparts versatility so they are good candidates to be used as a general starting material to anchor a group required for a specific synthetic purpose.

 Please wait while we load your content...
Please wait while we load your content...