Synthesis, biological evaluation and mechanism study of chalcone analogues as novel anti-cancer agents†
Abstract
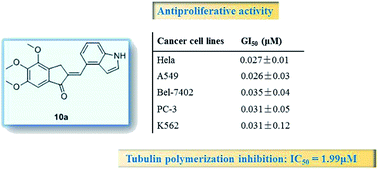
A series of novel chalcone analogues were designed, synthesized and evaluated as anticancer agents. The results of antiproliferative activity tests showed that most of the analogues exhibited moderate to very good antiproliferative activities with GI50 values in the micromol to sub-micromol range. Especially compound 10a gave 0.026 μM to 0.035 μM GI50 for five cancer cell lines. The mechanistic studies including tubulin polymerization inhibition, disruption of microtubule dynamics and cell cycle arrest assay demonstrated that compound 10a could effectively inhibit in vitro cellular tubulin polymerization, interfere with the mitosis, resulting in a prolonged G2/M cell cycle arrest and ultimately lead to cell apoptosis of cancer cells. Taken together, these results suggested that 10a may became a promising lead compound for development of new anticancer drugs.

 Please wait while we load your content...
Please wait while we load your content...