Design of chiral urea-quaternary ammonium salt hybrid catalysts for asymmetric reactions of glycine Schiff bases†
Abstract
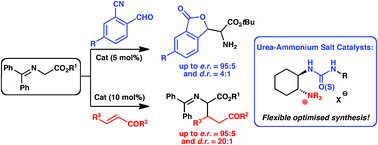
Bifunctional chiral urea-containing quaternary ammonium salts can be straightforwardly synthesised in good yield and with high structural diversity via a scalable and operationally simple highly telescoped sequence starting from trans-1,2-cyclohexanediamine. These novel hybrid catalysts were systematically investigated for their potential to control glycine Schiff bases in asymmetric addition reactions. It was found that Michael addition reactions and the herein presented aldol-initiated cascade reaction can be carried out to provide enantiomeric ratios up to 95 : 5 and good yields under mild conditions at room temperature.

 Please wait while we load your content...
Please wait while we load your content...