Design and discovery of tyrosinase inhibitors based on a coumarin scaffold†
Abstract
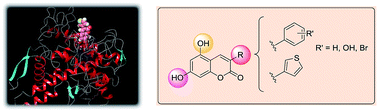
In this manuscript we report the synthesis, pharmacological evaluation and docking studies of a selected series of 3-aryl and 3-heteroarylcoumarins with the aim of finding structural features for the tyrosinase inhibitory activity. The synthesized compounds were evaluated as mushroom tyrosinase inhibitors. Compound 12b showed the lowest IC50 (0.19 μM) of the series, being approximately 100 times more active than kojic acid, used as a reference compound. The kinetic studies of tyrosinase inhibition revealed that 12b acts as a competitive inhibitor of mushroom tyrosinase with L-DOPA as the substrate. Furthermore, the absence of cytotoxicity in B16F10 melanoma cells was determined for this compound. The antioxidant profile of all the derivatives was evaluated by measuring radical scavenging capacity (ABTS and DPPH assays). Docking experiments were carried out on mushroom tyrosinase structures to better understand the structure–activity relationships.

 Please wait while we load your content...
Please wait while we load your content...