Synthesis of benzofulvenes through chemoselective Sonogashira and Barluenga couplings of ortho ethynyl-N-tosylhydrazones and cycloisomerization†
Abstract
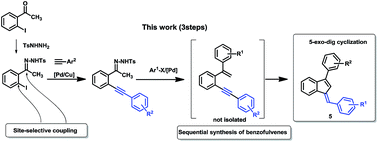
Chemoselective synthesis of ortho ethynylhydrazones 7 derived from 2-iodoacetophenone via a Sonogashira coupling is reported for the first time. In this challenging transformation, a site-selective coupling in the presence of a hydrazone function was developed. These compounds represent useful intermediates for the synthesis of benzofulvenes through a sequence of palladium-catalyzed cross-coupling and palladium-catalyzed 5-exo-dig cyclization.


 Please wait while we load your content...
Please wait while we load your content...