Performance of cobalt titanate towards H2O2 based catalytic oxidation of lignin model compound
Abstract
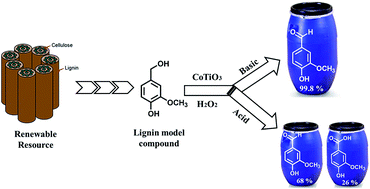
Mixed metal cobalt titanium oxide (CoTiO3) prepared by solution phase method has been evaluated for the liquid phase catalytic oxidation of vainlly alcohol to vanillin using H2O2 as an oxygen source. The morphology, phase composition and crystal structure of the freshly prepared and reused CoTiO3 catalyst was studied by SEM, EDX, XRD, XPS and Raman spectroscopy. Vanillyl alcohol conversion was influenced by various experimental conditions such as reaction time, temperature, molar ratio of reactants, catalyst loading, nature of solvent and reaction medium. The design a heterobimetallic oxide catalyst which can efficiently perform the high conversion and selective oxidation of vanillyl alcohol into fine chemicals, such as vanillin and vanillic acid. It has been found that during the 5 h reaction in NaOH, the CoTiO3 exhibits remarkable conversion of 99% and excellent selectivity of 99.8% to vanillin was achieved in acetic acid and isopropanol solvents, respectively. The oxidation reaction mechanism over the catalyst was postulated based on the observation product from the HPLC analysis. CoTiO3 catalyst can retain its performance without significant change in the catalytic activity after four consecutive cycles.

 Please wait while we load your content...
Please wait while we load your content...