Controlled electrodeposition of nanostructured Pd thin films from protic ionic liquids for electrocatalytic oxygen reduction reactions†
Abstract
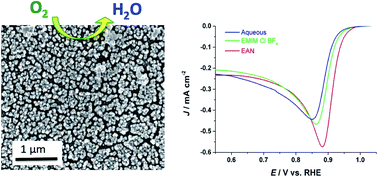
Palladium (Pd) has been widely used as electrocatalysts for a number of important electrochemical reactions. Herein, we report a facile electrodeposition method for fabrication of nanostructured Pd thin films from protic ionic liquids. The electrochemical behavior and electrodeposition of Pd were studied in a protic ionic liquid, ethylammonium nitrate (EAN), compared with aqueous solution and aprotic ionic liquids, 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate (EMIM-Cl-BF4), using cyclic voltammetry and chronoamperometry. The electrodeposition of Pd in protic ionic liquids is found to proceed via an instantaneous nucleation and diffusion-controlled 3D growth mechanism, and accompanied with hydrogen co-evolution at more negative deposition potentials. By controlling the electrodeposition media, electrodeposition potential and time, we show that Pd thin films can be electrodeposited from protic ionic liquids with finely tuned nanostructures and large surface area. The prepared Pd thin films were employed as electrocatalysts for oxygen reduction reactions in alkaline media with an onset potential of 0.95 V (RHE). It was found that Pd films prepared from protic ionic liquids exhibit larger electrochemically active surface area and higher catalytic activity for oxygen reduction reactions than aqueous and aprotic ionic liquid electrolytes under similar electrodeposition conditions.
- This article is part of the themed collection: Nanoscience and nanotechnology in electrochemistry

 Please wait while we load your content...
Please wait while we load your content...