New iodonium salts in NIR sensitized radical photopolymerization of multifunctional monomers†
Abstract
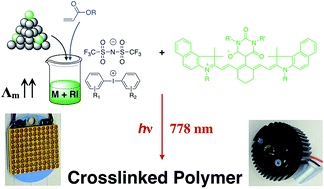
Reactivity of new iodonium salts [A-I-B]+X− was studied with near infrared (NIR) initiated radical polymerization by photo-DSC using the polymethine dye S1 (5-(6-(2-(3-ethyl-1,1-dimethyl-1H-benzo[e]indol-2(3H)-ylidene)ethylidene)-2-(2-(3-ethyl-1,1-dimethyl-1H-benzo[e]indol-3-ium-2-yl)vinyl)cyclohex-1-en-1-yl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyr-imidin-4-olate) as sensitizer. The iodonium salt [A-I-B]+X− functioned as a radical initiator bearing a different substitution pattern for the cation and the anion, respectively. Electron transfer of the excited state of S1 to [A-I-B]+X− (X−: benzilate, lactate, NO3−, PF6−, SbF6−, p-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ph-SO3−, p-C12H15-Ph-SO3−, CF3SO3−, C4F9SO3−, B(CN)4−, B(Ph)4−, B(PhF5)4−, N(CN)2−, (SO2-CF3)2N−) results in initiating radicals. The reactivity of S1/[A-I-B]+X− correlated with the conductivity of the salt in acrylate monomers such as hexane-1,6-diol diacrylate, tripropylene glycol diacrylate, poly(ethylene glycol) diacrylate and trimethylolpropane triacrylate. A high conductivity related always to a better reactivity in the monomer chosen. The solubility of [A-I-B]+X− determined ranged between several g L−1 up to well mixable systems (>2000 g L−1). Particular the bis(trifluoromethylsulfonyl) imide anion (N(SO2-CF3)2−) resulted in giant solubilities depending on the [A-I-B]+ cation. A high solubility did not always lead to a high reactivity. Furthermore, iodonium salts comprising the bis(trifluoromethylsulfonyl) imide anion exhibited a lower cytotoxicity compared to those with the tetraphenyl borate anion as determined by the MTT-test using CHO-9-cells.
CH-Ph-SO3−, p-C12H15-Ph-SO3−, CF3SO3−, C4F9SO3−, B(CN)4−, B(Ph)4−, B(PhF5)4−, N(CN)2−, (SO2-CF3)2N−) results in initiating radicals. The reactivity of S1/[A-I-B]+X− correlated with the conductivity of the salt in acrylate monomers such as hexane-1,6-diol diacrylate, tripropylene glycol diacrylate, poly(ethylene glycol) diacrylate and trimethylolpropane triacrylate. A high conductivity related always to a better reactivity in the monomer chosen. The solubility of [A-I-B]+X− determined ranged between several g L−1 up to well mixable systems (>2000 g L−1). Particular the bis(trifluoromethylsulfonyl) imide anion (N(SO2-CF3)2−) resulted in giant solubilities depending on the [A-I-B]+ cation. A high solubility did not always lead to a high reactivity. Furthermore, iodonium salts comprising the bis(trifluoromethylsulfonyl) imide anion exhibited a lower cytotoxicity compared to those with the tetraphenyl borate anion as determined by the MTT-test using CHO-9-cells.


 Please wait while we load your content...
Please wait while we load your content...