DMSO affects Aβ1–40's conformation and interactions with aggregation inhibitors as revealed by NMR†
Abstract
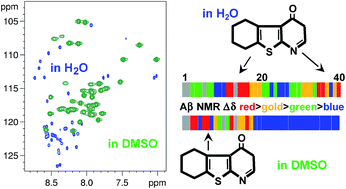
We show via 3D-heteronuclear NMR spectroscopy that Aβ1–40 adopts a disordered conformational ensemble with fluctuating turns in DMSOd6. Using NMR, we map the binding sites of three water-insoluble aggregation inhibitors to Aβ1–40 in DMSOd6 and discover remarkable differences in Aβ1–40 recognition by a fourth inhibitor in H2O versus DMSOd6.

 Please wait while we load your content...
Please wait while we load your content...