Synthesis and characterization of conjugated/cross-conjugated benzene-bridged boron difluoride formazanate dimers†
Abstract
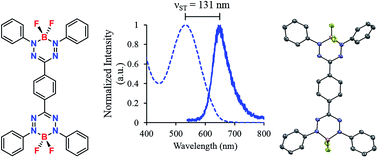
One of the most common strategies for the production of molecular materials with optical properties in the far-red/near-IR regions of the electromagnetic spectrum is their incorporation into dimeric architectures. In this paper, we describe the synthesis and characterization (1H, 11B, 13C and 19F NMR spectroscopy, IR and UV-vis absorption and emission spectroscopy, mass spectrometry and X-ray crystallography) of the first examples of boron difluoride (BF2) formazanate dimers. Specifically, the properties of meta- and para-substituted benzene-bridged dimers p-10 and m-10 were compared to closely related boron difluoride triphenyl formazanate complex 11 in order to assess the effect of electronic conjugation and cross conjugation on their light absorption/emission and electrochemical properties. While the properties of cross-conjugated dimer m-10 did not differ significantly from those of monomer 11, conjugated dimer p-10 exhibited red-shifted absorption and emission maxima and was easier to reduce electrochemically to its bis radical anion and bis dianion form compared to monomer 11. Both dimers are weakly emissive in the far-red/near-IR and exhibited large Stokes shifts (>110 nm, 3318 cm−1). Unlike a closely related para-substituted benzene-bridged boron dipyrromethene (BODIPY) dimer, the emission quantum yields measured for the BF2 formazanate dimers exceeded those observed for monomeric analogues.

 Please wait while we load your content...
Please wait while we load your content...