The growth of Nin clusters and their interaction with cubic, monoclinic, and tetragonal ZrO2 surfaces–a theoretical and experimental study†
Abstract
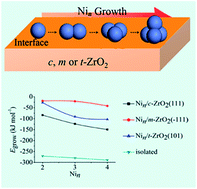
Ni/ZrO2 catalysts are widely used in many reactions such as CO/CO2 methanation and reforming of acetic acid. The kind of ZrO2 phase plays a vital role in the catalytic properties of Ni/ZrO2 catalysts that depend on the interface between zirconia and supported Ni particles. Periodic density functional theory was applied to systematically investigate the interaction of a single Ni atom and Nin (n = 2–4) clusters with cubic ZrO2 (c-ZrO2) (111), monoclinic ZrO2 (m-ZrO2) (−111), and tetragonal ZrO2 (t-ZrO2) (101) surfaces. Adsorption of the Ni atom and all Nin (n = 2–4) clusters on zirconium dioxide surfaces was kinetically and thermodynamically preferred. Adsorption of Nin clusters on the m-ZrO2(−111) surface is more stable than that on the t-ZrO2(101) surface, and the t-ZrO2(101) surface is more stable than the c-ZrO2(111) surface. The aggregation ability of Nin clusters on different ZrO2 surfaces and the isolated clusters follow the trend m-ZrO2(−111) < t-ZrO2(101) < c-ZrO2(111) < isolated cluster. Therefore, Nin clusters can have a better dispersion and can inhibit aggregation due to the support. What is more, the single-phase ZrO2 was synthesized and loaded with an equivalent content of active Ni components. The experimental results obtained by X-ray photoelectron spectroscopy analysis support the hypothesis that has been deduced.

 Please wait while we load your content...
Please wait while we load your content...