A new mechanistic insight on β-lactam systems formation from 5-nitroisoxazolidines†
Abstract
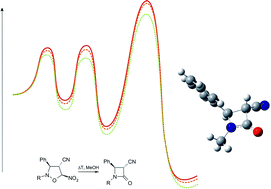
DFT calculations at various theory levels unequivocally show that conversion of 5-nitroisoxazolidines into β-lactams does not take place via a ring opening step (as was hitherto suggested), but takes place according to a three-step, domino-type reaction. Quantum-chemical studies do not indicate the existence of an alternative, kinetically allowed reaction mechanism. In addition, it was shown that this mechanism is a general one, and can be related to a wider class of compounds as an interesting alternative to popular synthesis methods of β-lactams.

 Please wait while we load your content...
Please wait while we load your content...