Chemical stability and tunable luminescence of Ln(iii)–K(i) coordination polymers featuring a tracery-like architecture†
Abstract
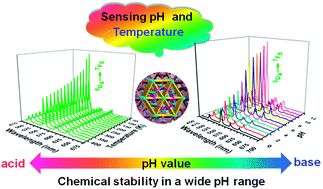
With the help of in situ generation of a sulfate anion, three heterometallic Ln(III)–K(I) sulfate and oxalate coordination polymers, formulated as [KLn(ox)(SO4)(H2O)] (ox = oxalate, Ln = Eu 1, Tb 2 and Dy 3) have been successfully synthesized and structurally characterized. They are isomorphic and each displays a tracery-like structure inlaid with two different kinds of hexagons. In addition, compound 2 is thermally stable up to 350 °C and exhibits unusual chemical stability in a wide pH range of 0–12, as confirmed by the variable temperature XRD and pH dependent XRD. Tunable luminescence of 2 is achieved by changing the temperature and pH, which indicates that it can be potentially used as a probe for sensing temperature and pH.


 Please wait while we load your content...
Please wait while we load your content...