Eutectic ionic liquid mixtures and their effect on CO2 solubility and conductivity†
Abstract
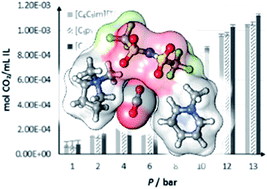
A simple binary system of compounds resembling short-chain versions of popular ionic liquids has been shown to have surprisingly complex properties. Combining methylated versions of pyridinium and pyrrolidinium bis[(trifluoromethyl)sulfonyl]imide gave desirable properties such as low viscosity and high conductivity solubility per unit volume. The binary combinations studied in this study showed that these materials were stable liquids at 50 °C and had a threefold improvement in conductivity over [C6C1im][Tf2N]. Despite the high densities of these materials, 2D-IR studies indicate increased ion mobility, likely due to the lack of hindering alkyl chains.


 Please wait while we load your content...
Please wait while we load your content...