Characterization and interfacial properties of the surfactant ionic liquid 1-dodecyl-3-methyl imidazolium acetate for enhanced oil recovery
Abstract
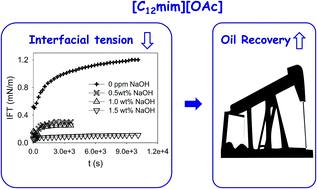
The use of surfactant ionic liquids has recently gained attention for surfactant-based enhanced oil recovery. The introduction of the surfactant character in ionic liquids combines interesting properties of both types of chemicals, which are relevant for this application. An imidazolium-based surfactant has been synthesized combined with an acetate counter-ion. The aggregation effects in water were evaluated by means of surface tension and electrical conductivity. The dynamic interfacial tensions between the aqueous solutions of the surfactant ionic liquid and crude oil (Saharan blend) were evaluated by the spinning drop method. The effect of different variables has been analyzed, namely the concentration of surfactant ionic liquid, electrolytes (NaCl) and alkalis (NaOH, Na2CO3), and temperature. Formulations of ionic liquids and alkalis were tested for the first time for enhanced oil recovery. The results obtained here improve significantly (at least one order of magnitude, most often two) previous results obtained up to now solely with ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...