One-pot chemo/regio/stereoselective generation of a library of functionalized spiro-oxindoles/pyrrolizines/pyrrolidines from α-aroylidineketene dithioacetals†
Abstract
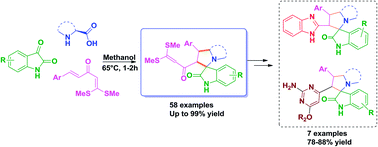
An efficient chemo/regio/stereoselective synthesis of novel and functionalized spiro-oxindole/pyrrolizine/pyrrolidine scaffolds has been achieved. The in situ generated azomethine ylide from isatin & L-proline/phenyl alanine underwent 1,3-dipolar cycloaddition with α-aroylidineketene dithioacetals under simple reaction conditions affording spiro-oxindole derivatives. This protocol exhibits an interesting double bond selectivity of α-aroylidineketene dithioacetals. Furthermore, utilizing this spiro-oxindoles scaffold, biologically important benzimidazole and pyrimidine based poly heterocycles were also synthesized.

 Please wait while we load your content...
Please wait while we load your content...