Al(CH3)3-promoted Pt/MCM-41 catalysts for tetralin hydrogenation in the presence of benzothiophene and promotion mechanism of Al-promoted Pt/MCM-41 catalysts
Abstract
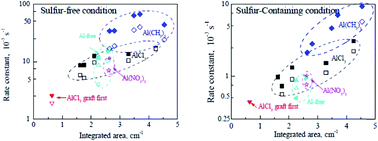
Al(CH3)3-promoted Pt–Al/MCM-41 catalysts with Al/Pt ratios from 0 to 20 were prepared for tetralin hydrogenation under sulfur-free and sulfur-containing conditions. NH3-TPD and Py-FTIR results indicate that the amount of acid catalyst increases with increasing Al/Pt. The electron withdrawing effect of the Al-promoter decreases the electron density on the platinum particles and leads to the formation of electron deficient Ptδ+. The isolation effect of the Al-promoter, which benefits platinum dispersion, plays a leading role at low Al/Pt, while the anchor effect, which leads to large platinum particles, dominates at high Al/Pt. Platinum dispersion increases at low Al/Pt and decreases at high Al/Pt. The catalyst with Al/Pt = 10 has the best platinum dispersion. All Al-promoted catalysts show much better tetralin hydrogenation activity and sulfur-tolerance than the Al-free catalyst, and the catalyst with Al/Pt = 10 is the best. The improvement in platinum dispersion is the primary factor that benefits both tetralin hydrogenation performance and sulfur tolerance. However, tetralin hydrogenation prefers less electron deficient platinum particles while sulfur tolerance favors more electron deficient Ptδ+.

 Please wait while we load your content...
Please wait while we load your content...