Synthesis and thermal gelation of hydroxypropyl chitin
Abstract
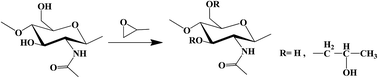
Fully water-soluble hydroxypropyl chitin (HPCh) was synthesized by the modification of chitin with propylene oxide in aqueous NaOH solution, a green and good solvent to chitin. HPChs with different degrees of substitution (DS) were obtained by varying the feeding ratio of propylene oxide to chitin. The HPCh solutions undergo a sol-to-gel transition upon heating. The transition was reversible, although an apparent hysteresis occurs in the cooling process. The gelation temperature (Tgel) decreases with increasing DS and concentration of the polymer. The thermal gelation could occur even at a concentration as low as 0.25 wt%. By adjusting the DS of the polymer, Tgel could be tuned from ∼20 °C to ∼80 °C. Meanwhile NaCl concentration and pH only slightly influence the Tgel. Preliminary tests show that the polymer is non-toxic to cells and it degrades in the presence of lysozyme. The ability to gel at temperatures below the body temperature, at relatively low polymer concentration, and biocompatibility and biodegradability of HPCh make the new thermal gelling materials quite suitable for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...