Oxidative coupling of 2-naphthol to (R)/(S)-BINOL by MCM-41 supported Mn-chiral Schiff base complexes†
Abstract
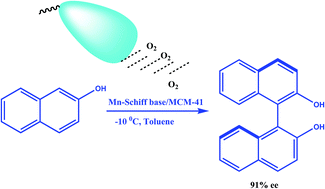
Three Mn(III)-chiral Schiff base complexes supported on MCM-41 are found to be effective reusable catalysts for enantioselective oxidation of 2-naphthol to (R)- and (S)-BINOL (1,1′ bi-2-naphthol) in the presence of oxygen. The supported Mn(III)-complexes are characterized by PXRD, FTIR, solid state-NMR, BET, and cyclic voltammetry study. The homo-coupling reaction with oxygen as the oxidant is promoted by 20 mg of Mn(III) Schiff base complexes to afford binaphthols in nearly quantitative yields with high enantioselectivity of up to 91% ee. The catalytic activities of the homogeneous and heterogeneous chiral catalyst are found to be almost similar. However, the heterogeneous counterparts are found to be advantageous in terms of recyclability and storability. Oxygen partial pressure, the nature of the solvent, temperature and the amount of catalyst affect the catalytic oxidation process. High temperature and highly polar solvent are found to have adverse effects on the catalytic oxidation process.

 Please wait while we load your content...
Please wait while we load your content...