Unravelling the mechanism of the ketene-imine Staudinger reaction. An ELF quantum topological analysis†
Abstract
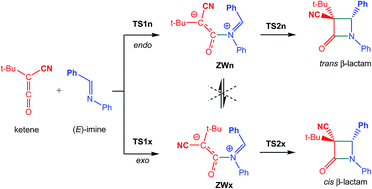
The mechanism of the ketene-imine Staudinger (KI-S) reaction between t-butyl-cyano ketene and N-phenyl phenylimine has been studied using DFT methods at the MPWB1K/6-311G(d) computational level. The reaction takes place through a two-step mechanism: (i) the first step is the nucleophilic attack of the imine nitrogen lone pair on the central carbon of the ketene yielding a zwitterionic (ZW) intermediate; (ii) the second step, which is the rate- and stereoselectivity-determining step, is a ring-closure process achieved by a nucleophilic attack of the terminal carbon atom of the ketene on the imine carbon atom. Due to the unfeasibility of a cis/trans and an E/Z stereoisomerisation at the ZW intermediates, trans and cis β-lactams are formed along the endo and exo stereoisomeric channels, respectively. An electron localisation function (ELF) quantum topological analysis of the bonding changes along the KI-S reaction permits a complete characterisation of the mechanism. The first step is associated with the formation of the N1–C4 single bond along the nucleophilic attack of the imine nitrogen lone pair on the central carbon of the ketene, while the second step is associated with a ring-closure process achieved by the C-to-C coupling of the C2 and C3 pseudoradical centers generated in the previous phases. The present theoretical study makes it possible to reject those analyses based on the FMO theory, in which HOMO/LUMO interactions along the nucleophilic attack of the imines on the ketenes and a feasible torquoelectronic effect along the conrotatory ring-closure step control the cis/trans stereoselectivity in the formation of β-lactams.

 Please wait while we load your content...
Please wait while we load your content...