Molecular docking studies and biological evaluation of chalcone based pyrazolines as tyrosinase inhibitors and potential anticancer agents
Abstract
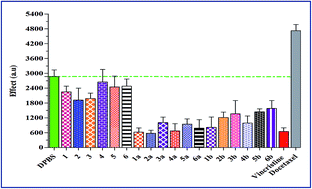
Studies on the discovery of tyrosinase enzyme inhibitors and exploration for better cytotoxic agents remain an important line in drug discovery and development. A series of synthetic chalcones and pyrazoline derivatives was evaluated for their inhibitory effects on the diphenolase activity of mushroom tyrosinase. The effects of these compounds on proliferation and microtubule assembly were also evaluated in seven different cancer cell lines. The results revealed that some of the synthetic compounds showed significant inhibitory activity, with four compounds being more potent tyrosinase inhibitors than the reference standard inhibitor kojic acid. Several compounds were toxic to cancer cell lines. Compound 1a was found to possess the highest anticancer activity towards all cell lines with an IC50 in the range of 0.9–2.2 μM. Seven of the compounds showed considerable tubulin polymerization activity at a concentration of 25 μM. Molecular modeling studies of these synthetic compounds were performed to investigate their interactions with the tyrosinase enzyme. The structure–activity relationship (SAR) study using in-silico analysis matched well with the in vitro tumour cell inhibitory activity.
- This article is part of the themed collection: Molecular modelling


 Please wait while we load your content...
Please wait while we load your content...