An approach to asymmetric synthesis of β-aryl alanines by Pd(0)-catalyzed cross-coupling and cyanate-to-isocyanate rearrangement†
Abstract
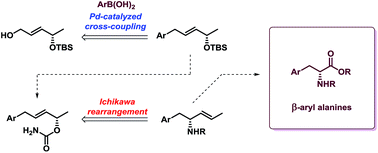
A new method for the asymmetric synthesis of β-aryl alanines is reported. The developed strategy is based on Pd(0)-catalyzed cross-coupling of chiral allyl alcohols with arylboronic acids followed by Ichikawa sigmatropic rearrangement of allyl cyanates to isocyanates. In the rearrangement step the allyl alcohol is stereospecifically transformed into the corresponding allylamine with complete chirality transfer. The resulting allylamines are converted into β-aryl alanines after oxidation of the double bond.

 Please wait while we load your content...
Please wait while we load your content...