Enzymology and thermal stability of phytase appA mutants†
Abstract
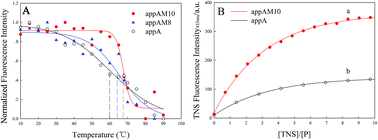
Escherichia coli phytase appA which hydrolyzes phytate has been widely applied as an important feed supplement, but the need to improve the thermal tolerance remains. Here, ten residue substitutions (W46E, Q62W, A73P, K75C, S146E, R159Y, N204C, Y255D, Q258N and Q349N) were introduced to enhance its thermal tolerance. Results showed the purified appAM10 had a specific activity of 3022 U mg−1 with an MW of approximately 53–55 kDa. Compared with the non-engineered enzyme produced by appA (GenBank Accession DQ513832), appAM10 showed an enhancement in thermal tolerance and 7.5 °C increased in the melting temperatures (Tm). To understand the mechanism of the improvement in its thermal tolerance, conformational changes between appA and its mutant appAM10 have been investigated in detail by means of fluorescence spectroscopy. The results indicated that appAM10 had enhancement of α-helix content and displayed greater exposed hydrophobic surface than appA. These conformational changes made the appAM10 more stable against heat treatment. This study provided a biotechnologically useful mutant and expanded our knowledge about the mechanisms of phytase thermal tolerance.

 Please wait while we load your content...
Please wait while we load your content...