A pair of Schiff base enantiomers studied by absorption, fluorescence, electronic and vibrational circular dichroism spectroscopies and density functional theory calculation†
Abstract
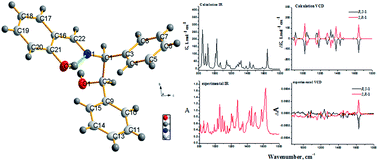
2-(((1R,2S)-2-Hydroxy-1,2-diphenylethylimino)methyl)phenol and 2-(((1S,2R)-2-hydroxy-1,2-diphenylethylimino)methyl)phenol have been synthesized as a pair of enantiomeric Schiff bases of a chiral salicylaldehyde. These compounds were subsequently characterized by melting point, EI-MS, IR, 1H-NMR and X-ray crystallographic analyses. The UV-vis absorption, fluorescent emission, electronic and vibrational circular dichroism (ECD and VCD) spectral properties of these enantiomers were determined in solution in a variety of different solvents, including acetonitrile, ethanol and hexane, as well as being determined in the solid state. The effects of the different solvents were evaluated in detail, and it was found that the enol-imine tautomer existed as the dominant species in nonpolar solvents, such as hexane, and that the enol-imine and keto-enamine tautomers coexisted in polar solvents, such as ethanol. Theoretical IR and VCD spectra of the enantiomers were calculated at the B3LYP/6-311+G(d,p) level by density functional theory. Given that the different tautomers coexisted in solution and VCD is very sensitive to conformational changes, it was not possible to reliably determine the absolute configurations of the enantiomers based on their solution phase VCD spectra. However, the calculated IR and VCD spectra in a vacuum were in good agreement with the experimental solid state spectra, and it was therefore possible to reasonably assign the absolute configurations of the enantiomers based on the VCD calculations. This study therefore represents a good example of the practical application of solid state VCD spectra to assign the absolute configurations of different enantiomers.

 Please wait while we load your content...
Please wait while we load your content...