Stabilization of η3-indenyl compounds by sterically demanding N,N-chelating ligands in the molybdenum coordination sphere†
Abstract
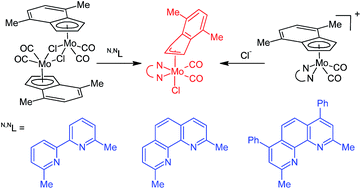
A series of η3-indenyl molybdenum compounds [(η3-4,7-Me2C9H5)Mo(CO)2(N,NL)Cl] (N,NL = bpy, phen, pyma), isostructural with well-known η3-allyl compounds, was synthesized from the recently established halide synthon [{(η5-4,7-Me2C9H5)Mo(CO)2(μ-Cl)}2]. The low stability of the hexacoordinated η3-indenyl molybdenum species in solution has been overcome by a modification of the chelating ligand. Hence, the dissociation of the compounds bearing ligands with methyl groups beside nitrogen donor atoms (e.g. 6,6′-Me2-bpy, 2,9-Me2-phen; 2,9-Me2-4,7-Ph2-phen) is strongly disfavored due to the steric requirements of the substituents. The considerable discrimination of the pentacoordinated species enables the use of [(η5-4,7-Me2C9H5)Mo(CO)2(2,9-Me2-phen)][BF4] for the assembly of derivatives bearing other halides and pseudohalides in the coordination sphere of molybdenum. The current study further describes some other new indenyl complexes accessible from [{(η5-4,7-Me2C9H5)Mo(CO)2(μ-Cl)}2]. All structural types presented in this experimental study were supported by X-ray crystallographic data.

 Please wait while we load your content...
Please wait while we load your content...