Polyelectrolytes in dilute solution: viscometric access to coil dimensions and salt effects
Abstract
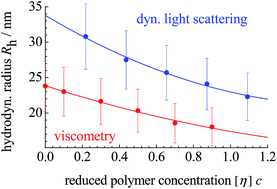
Copolymers of acrylamide (AM) and diallyldimethylammonium chloride (DADMAC), differing in molar masses M (52.3 to 227 kDa) and degrees of charging y (0.2 to 0.6), were studied with respect to their viscometric behavior in dilute aqueous solutions containing variable amounts of NaCl. Complementary measurements were performed on a Brookhaven 90 plus particle size analyzer. M dominates the intrinsic viscosities [η]. For the viscometric interaction parameters B this is only true for large concentrations of extra salt. [η] and B as a function of solvent salinity follow Boltzmann laws. Coil dimensions, determined either via dynamic light scattering or viscometry, agree well. For low salt contents of the solvent the radii decrease with rising polymer concentration, whereas they increase for high salt concentrations; at a characteristic salinity of the solvent they become independent. Zeta potentials grow from +20 to +40 mV as y goes up.

 Please wait while we load your content...
Please wait while we load your content...