Pyridylpentazole and its derivatives: a new source of N5−?†
Abstract
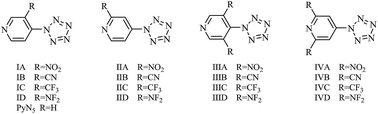
Pyridylpentazole (PyN5) and its derivatives with 1–2 electron withdrawing groups (–NO2, –CN, –CF3 and –NF2) were studied using density functional theory to assess their potentials as the source of pentazole anion N5− for replacement of phenylpentazole (PhN5). N5− can be produced more easily from PyN5s because the activation energies (Ea,1 = 364.7–387.1 kJ mol−1) for the cleavage of the central C–N bonds of PyN5s are lower than that of PhN5 (395.3 kJ mol−1) and the C–N bond decomposition reactions of the former are faster than that of the latter. The energies (Ea,2) required for dissociation of the N5 ring of PyN5s (67.6–84.1 kJ mol−1) are smaller than that of PhN5 (88.7 kJ mol−1), and the rates of the former are faster than that of the latter. Comparing the stabilities of the C–N bond and the N5 ring of PyN5s and PhN5, the decrement in the C–N bond stability (2.1–7.8%) caused by the pyridine ring and substituents is less obvious than that of the N5 ring (12.7–23.7%). Although N5− can be obtained more easily and faster from PyN5s than PhN5, the smaller Ea,2 makes PyN5s less stable than PhN5, so pyridylpentazoles may not be a better source of N5− than phenylpentazole.

 Please wait while we load your content...
Please wait while we load your content...