Synthesis of some novel steroidal 1,2,4,5-tetraoxanes
Abstract
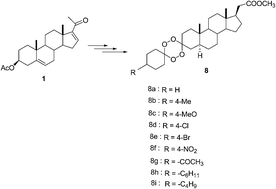
A facile synthesis of A-ring manipulated C-20 methyl carboxylate steroid derivative with unsymmetrical dispiro 1,2,4,5-tetraoxanes has been focused herein via acid catalyzed cyclocondensation of bis-epidioxy ketone. Novel stable unsymmetrical steroidal based spirocycloalkane 1,2,4,5 tetraoxane 8 has been developed from 3β-acetoxy-pregn-5(6), 16(17)-diene-20-one (16-dehydropregnenolone acetate, i.e. 16-DPA) 1 via metal-mediated halogenation as a key reaction.

 Please wait while we load your content...
Please wait while we load your content...