Tetrakis-imidazolium and benzimidazolium ionic liquids: a new class of biodegradable surfactants†
Abstract
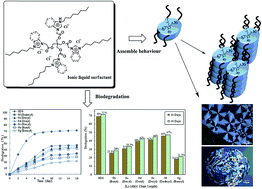
Novel series of tetra-cationic ionic liquids containing alkyl or phenyl side chains and ester groups within the same molecule were successfully prepared. Based on imidazolium and benzimidazolium, these ionic liquids were synthesized from readily available starting materials in high yield. Surfactant properties including liquid crystalline behaviour and surface properties as well as their biodegradability were investigated. Tetrakis-imidazolium ionic liquid compounds showed assembly behaviour in the pure form (i.e. spontaneously) and in the presence of polar or nonpolar solvents, while both imidazolium and benzimidazolium ionic liquids effectively reduced the surface tension of water in the range of 29–34 mN m−1. The incorporation of tetra alkyl or phenyl side chains into imidazolium and benzimidazolium ionic liquids with tetra-ester groups, significantly improved the biodegradation. ‘Closed-Bottle Test’ OECD 301D and sodium n-dodecyl sulphate (SDS) as a reference were used for evaluation. The linear alkyl side chains (i.e. butyl, hexyl, octyl, decyl and dodecyl) in both tetrakis-imidazolium and benzimidazolium ionic liquids promote the increasing in biodegradation and phase behaviour results comparing to aromatic side-chains.

 Please wait while we load your content...
Please wait while we load your content...