Enantiomeric neolignans and sesquineolignans from Jatropha integerrima and their absolute configurations†
Abstract
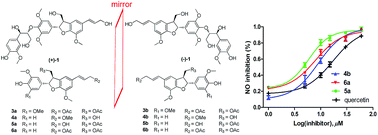
Two pairs of new sesquineolignan enantiomers, (±)-jatrointelignans A and B (1a/1b and 2a/2b), one pair of new neolignan enantiomers, (±)-jatrointelignan D (4a/4b), and two new neolignans, (+)-jatrointelignan C (3a), and (+)-schisphenlignan I (5a) together with seven known analogues (3b, 5b, 6a, 6b, 7a, 7b, and 8) were isolated from the trunks of Jatropha integerrima. The structures were determined by combined spectroscopic and chemical methods, and their absolute configurations were elucidated by the circular dichroism (CD) method, in particular the configurations of the aryl glycerol-7′′,8′′-yloxy moiety in 1 and 2 were determined via the Rh2(OCOCF3)4-induced CD analysis. All compounds were examined for their inhibitory effects on nitric oxide (NO) production induced by lipopolysaccharide (LPS) in BV-2 microglial cells, and compounds 5a, 6a, and 4b exhibited pronounced inhibition on NO production with IC50 values in the range of 5.9–8.9 μM, being more active than the positive control, quercetin (IC50 = 17.0 μM).

 Please wait while we load your content...
Please wait while we load your content...