Application of electrodialysis to remove copper and cyanide from simulated and real gold mine effluents
Abstract
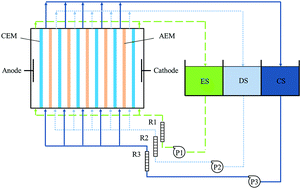
In this study, a laboratory-scale electrodialysis (ED) system with an effective area of 88 cm2 was used to remove copper and cyanide in simulated and real gold mine effluents. The membrane fouling was characterized by FTIR, SEM-EDX, membrane resistance measurements and static contact angle. The effects of applied voltage, initial concentration, and flux rate on the removal rate of copper and cyanide were investigated. The highest copper (99.41%) and cyanide (99.83%) removal rates were achieved under the following conditions: applied voltage of 25 V, initial concentration of C2 (concentration of copper and cyanide were 47 mg L−1 and 242 mg L−1), and a flux rate of 4.17 mL s−1. In addition, the lowest concentration of copper (0.44 mg L−1), cyanide (0.48 mg L−1) and zinc (0.34 mg L−1) in the treated effluent were all below regulatory limits (copper, cyanide <0.5 mg L−1, zinc <2.0 mg L−1). The results showed the presence of CuCN, [Cu(CN)3]2−, Cu(OH)2, and Zn(OH)2 in the precipitate, and the fouling of anion-exchange membranes (AEMs) could be decreased significantly via pH adjustment. This research provides a new insight into the removal of copper and cyanide from gold mine effluent.

 Please wait while we load your content...
Please wait while we load your content...