Decarboxylative acylation of arenes with mandelic acid derivatives via palladium-catalyzed oxidative sp2 C–H activation
Abstract
An efficient palladium catalyzed decarboxylative acylation of arenes with mandelic acid derivatives via oxidative sp2 C–H activation in the presence of tert-butyl hydroperoxide has been developed. The acylation reaction is assisted by a pyridine directing group. The starting materials are inexpensive and readily available. This method provides an economical and convenient way to synthesize aryl ketones.
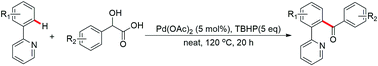

 Please wait while we load your content...
Please wait while we load your content...