Pt nanoparticles on tin oxide based support as a beneficial catalyst for oxygen reduction in alkaline solutions
Abstract
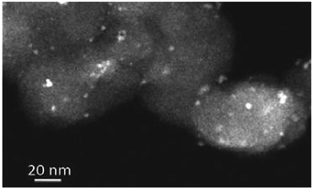
A platinum nanocatalyst on Sb doped tin oxide support (Sb–SnO2) was synthesized and characterized as a catalyst for oxygen reduction reaction in 0.1 mol dm−3 NaOH solution at 25 °C. Sb (5%) doped tin oxide support was synthesized by a modified hydrazine reduction procedure. The platinum nanocatalyst (20% Pt) on Sb–SnO2 support was synthesized by a borohydride reduction method. The synthesized support and catalyst were characterized by high resolution transmission electron microscopy (HRTEM) and X-ray photoelectron spectroscopy (XPS) and X-ray diffraction technique (XRD). X-ray photoelectron spectroscopy was applied to characterize the chemical status of elements before and after Pt-treatment. XPS spectra of Sn 3d, Pt 4f, Sb 3d and O 1s revealed that the Pt-deposition on Sb–SnO2 support induced the reduction of the Sn(4+) oxidation state to Sn(2+) and Sn(0) states, while Pt remained in the metallic state and Sb was in the (3+) oxidation state. Homogenous Pt nanoparticle distribution over the support, without pronounced particle agglomeration, was confirmed by HRTEM technique. The average Pt particle size was 2.9 nm. The electrochemically active Pt surface area of the catalyst was determined by the integration of the cyclic voltammetry curve in the potential region of underpotential deposition of hydrogen, after double layer charge correction, taking into account the reference value of 210 μC cm−2 for full monolayer coverage. This calculation gave the value of 51 m2 g−1. The kinetics of the oxygen reduction reaction with Pt/Sb–SnO2 catalyst was studied by cyclic voltammetry and linear sweep voltammetry using a rotating gold disc electrode. Two different Tafel slopes were observed: one close to 60 mV dec−1 in the low current density region, and another at ∼120 mV dec−1 in the higher current densities region, as was already referred in previous reports for the oxygen reduction reaction with polycrystalline Pt, as well as with different Pt based nanocatalysts. The specific activities for oxygen reduction, expressed in terms of kinetic current densities per electrochemically Pt active surface area, as well as per mass of Pt loaded, at the constant potential of practical interest (0.85 V and 0.90 V vs. RHE), were compared to a carbon supported (Vulcan XC-72) catalyst. The Pt/Sb–SnO2 catalyst exhibited similar catalytic activity for oxygen reduction reaction like carbon supported one. The advantages of the carbon free support application in terms of the durability and stability of the catalysts were proved by accelerated stability tests.

 Please wait while we load your content...
Please wait while we load your content...