Highly efficient click reaction on water catalyzed by a ruthenium complex†
Abstract
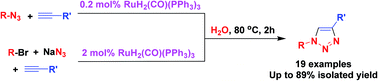
The highly efficient click reaction between terminal alkynes and azides has been achieved on water using ruthenium complex RuH2(CO)(PPh3)3 as the catalyst, and the catalyst loading was decreased to 0.2 mol% on water from 5 mol% in organic solvent. The RuH2(CO)(PPh3)3/H2O system also catalyzed the one-pot click reaction of bromides, sodium azide and alkynes; in this process, azides formed in situ and then underwent a click reaction with alkynes. In both aqueous processes, 1,4-disubstituted 1,2,3-triazoles were obtained in 50–89% yield with high regioselectivity.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...