Nitric oxide release by N-(2-chloroethyl)-N-nitrosoureas: a rarely discussed mechanistic path towards their anticancer activity†
Abstract
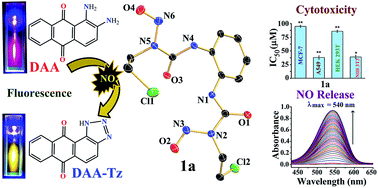
N-(2-Chloroethyl)-N-nitrosoureas (viz. carmustine (BCNU), lomustine) are used in critical cases of brain tumors or leukemia since these compounds are very toxic in nature. The mechanistic pathways of these compounds project the generation of isocyanates along with [–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–CH2–CH2–Cl]+OH− which, due to its reactivity, will breakdown further to produce DNA adducts and various other metabolites. Almost all of the literature predicting the mechanism does not discuss nitric oxide (NO) release as one of the viable mechanistic pathways for these N-(2-chloroethyl)-N-nitrosoureas. Our studies with two aromatic N-(2-chloroethyl)-N-nitrosoureas show that NO release may be a facile pathway for their activity. We have probed the NO release through fluorescence and the traditional Griess reagent assay. The aqueous stability studies show that the rates of decomposition of the two aromatic N-(2-chloroethyl)-N-nitrosoureas are of the order of 10−2 min−1. Initial studies of the cytotoxicity against two different cancer cell lines show that they are quite efficient even under hypoxic conditions, compared to the clinical compound BCNU, based on the results on human breast (MCF-7) and lung (A549) adeno carcinoma cell lines. The IC50 values range from about 38–95 μM. Encouragingly the 1-(4-chloro-1,2-phenylene)bis(3-(2-chloroethyl)-3-nitrosourea) (2a) is selectively more toxic to the A549 cancer cells (IC50 = 41 ± 4 μM) than the normal mouse embryonic fibroblast (NIH3T3, IC50 63 ± 4 μM) or non-tumorigenic human embryonic kidney cell line (HEK293T, IC50 = 83 ± 2 μM) and arrests the cell cycle in the G2/M-phase in the A549 cell line. Even under hypoxic conditions compound 2a is active at 49 ± 5 μM.
N–CH2–CH2–Cl]+OH− which, due to its reactivity, will breakdown further to produce DNA adducts and various other metabolites. Almost all of the literature predicting the mechanism does not discuss nitric oxide (NO) release as one of the viable mechanistic pathways for these N-(2-chloroethyl)-N-nitrosoureas. Our studies with two aromatic N-(2-chloroethyl)-N-nitrosoureas show that NO release may be a facile pathway for their activity. We have probed the NO release through fluorescence and the traditional Griess reagent assay. The aqueous stability studies show that the rates of decomposition of the two aromatic N-(2-chloroethyl)-N-nitrosoureas are of the order of 10−2 min−1. Initial studies of the cytotoxicity against two different cancer cell lines show that they are quite efficient even under hypoxic conditions, compared to the clinical compound BCNU, based on the results on human breast (MCF-7) and lung (A549) adeno carcinoma cell lines. The IC50 values range from about 38–95 μM. Encouragingly the 1-(4-chloro-1,2-phenylene)bis(3-(2-chloroethyl)-3-nitrosourea) (2a) is selectively more toxic to the A549 cancer cells (IC50 = 41 ± 4 μM) than the normal mouse embryonic fibroblast (NIH3T3, IC50 63 ± 4 μM) or non-tumorigenic human embryonic kidney cell line (HEK293T, IC50 = 83 ± 2 μM) and arrests the cell cycle in the G2/M-phase in the A549 cell line. Even under hypoxic conditions compound 2a is active at 49 ± 5 μM.

 Please wait while we load your content...
Please wait while we load your content...