Effect of precursor on the formation of different phases of iron oxide nanoparticles
Abstract
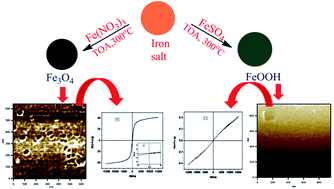
Trioctylamine is known to act simultaneously as a reducing as well as a hydrolyzing agent. In this work, we elucidate the effect of precursors in the formation of different forms of iron oxide and oxyhydroxide by using trioctylamine in a simple reflux process. A series of primary (octylamine), secondary (dioctylamine (DOA)) and tertiary (trioctylamine (TOA)) amines have also been examined. The obtained nanoparticles were characterized by FT-IR (Fourier transform infrared spectroscopy), XRD (X-ray diffraction), TEM (transmission electron microscopy), VSM (vibrating sample magnetometry) and Mössbauer spectroscopy. It was found that when ferric salts were used, the spinel form of iron oxide was formed whereas ferrous salts resulted in the formation of oxyhydroxide. In the presence of NO3− ions, the magnetite phase was predominantly formed using trioctylamine while a mixture of the magnetite and lepidocrocite forms of iron oxide was obtained in dioctylamine and lepidocrocite precipitated using octylamine.

 Please wait while we load your content...
Please wait while we load your content...