Rapid decomposition of Direct Blue 6 in neutral solution by Fe–B amorphous alloys
Abstract
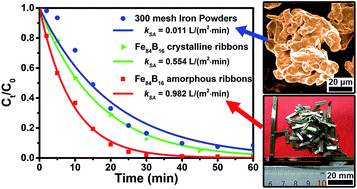
The development of environmental-friendly materials for the highly-efficient purification of dye-containing wastewater is important and attractive. In this work, Fe–B amorphous alloy ribbons were fabricated and employed to degrade the azo dye, Direct Blue 6. It shows that Fe–B binary amorphous alloys possess low reaction activation energy (25.43 kJ mol−1) and much higher degrading efficiency than their crystalline counterparts and the commercial iron powders. Under the same experimental conditions (25 °C, initial pH = 7), the surface area normalized reaction rate constant of Fe84B16 amorphous alloy is approximately 1.8 and 89 times as that of its crystalline alloy and the 300 mesh iron powders, respectively. It indicates that the homogeneous amorphous structure is beneficial to the degradation rate of the zero valent iron. It has been found that boron plays an important role in enhancing the degradation rate because it contributes to the formation of an incompact oxide layer at the metal–water interface. Unlike the dense oxide layer formed on the surface of iron powders, this incompact layer would benefit iron atoms' movement and promote the reduction of contaminants. The present results provide a new amorphous zero valent iron material and a new way of designing proper iron-based alloys for waste-water remediation.

 Please wait while we load your content...
Please wait while we load your content...