Abstract
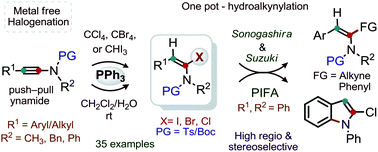
In this study, we demonstrate metal free regio- and stereoselective α-halogenation (Cl, Br and I) of ynamides. The halogenation of ynamides in the presence of PPh3 and CCl4, CBr4 or CHI3 in moist CH2Cl2 at room temperature provides good-to-excellent yields of a wide variety of stable (E)-α-haloenamides; thus, the reaction has a broad scope. Chlorination of ynamides at room temperature is particularly notable. The sequential one pot α-iodination and alkynylation of ynamides provide synthetically useful amidoenynes. The chlorination of 3-acetoxy ynamide delivers 3-acetoxy-α-chloro enamide without the migratory-assistance of the acetoxy moiety.

 Please wait while we load your content...
Please wait while we load your content...