C–H insertions in oxidative gold catalysis: synthesis of polycyclic 2H-pyran-3(6H)-ones via a relay strategy†
Abstract
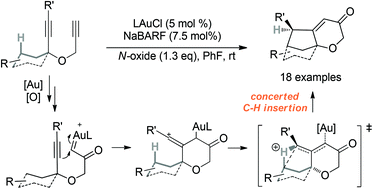
An expedient synthesis of bicyclic/polycyclic 2H-pyran-3(6H)-ones is realized in a cascade cyclization triggered by oxidative gold catalysis and terminated by streamlining C–H insertions. In this reaction, an α-oxo gold carbene intermediate, initially formed upon gold-catalyzed oxidation of alkynes, could be trapped by a tethered C–C triple bond, thereby resulting in the formation of a putative vinyl cation intermediate. This intermediate of high electrophilicity is proposed to be responsible for intramolecular C–H insertions, the mostly concerted nature of which is established by the reactions of chiral substrates. The reaction provides a rapid approach to the construction of functionalized polycyclic systems from easily accessible bispropargyl ethers and with minimal structural prefunctionalization.
- This article is part of the themed collection: Carbenes for Today

 Please wait while we load your content...
Please wait while we load your content...