Enantiodiscrimination of carboxylic acids using the diphenylprolinol NMR chiral solvating agents†
Abstract
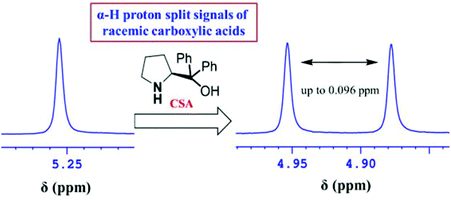
Enantiopure diphenylprolinols were synthesized from a commercially available starting material. The utility of 1H NMR spectroscopy for the differentiation of enantiomers using these chiral compounds as CSAs is stated, and their capacity acting as receptors for various carboxylic acids via hydrogen bonding is exploited. A linear relationship has been observed between the experimental and observed values of ee indicating the possible use of these compounds for quick and reliable analysis of enantiomerically enriched samples of various mandelic acids. From the experiments performed a preliminary conclusion indicated that the diphenylprolinol 1b with the free NH and OH is most effective in the chiral discrimination of carboxylic acids in 1H NMR.

 Please wait while we load your content...
Please wait while we load your content...