Alkylation/1,2-aryl migration of α-aryl allylic alcohols with α-carbonyl alkyl bromides using visible-light photoredox catalysis†
Abstract
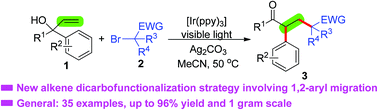
A novel visible-light-induced alkene difunctionalization strategy is described for the synthesis of 1,5-dicarbonyl compounds from two reaction partners, α-aryl allylic alcohols and α-carbonyl alkyl bromides. This method is successful by sequential alkylation of an alkene C–C double bond and intramolecular 1,2-aryl migration, and shows a broad substrate scope, excellent functional group tolerance and good selectivity.

 Please wait while we load your content...
Please wait while we load your content...