Successive Cu/Pd transmetalation relay catalysis in stereoselective synthesis of tetraarylethenes†
Abstract
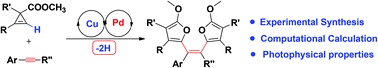
A new and efficient strategy for the synthesis of tetraaryl-substituted olefins has been developed. With a Cu/Pd-catalyzed isomerization/insertion/oxidative coupling cascade reaction of cyclopropene with internal alkynes, a wide variety of cis-tetrasubstituted olefins were synthesized in good yields as single stereoisomers. The photophysical properties of these novel tetraarylethenes were fully characterized and proved to be good AIE (aggregation-induced emission) luminogens. Experimental studies and theoretical calculation indicated that Cu(I) and Pd(II) were the actual catalysts. A novel deprotonative Cu-catalyzed cyclopropene cycloisomerization and subsequent successive Cu/Pd transmetalation relay mechanism was proposed for the discovered reaction.

 Please wait while we load your content...
Please wait while we load your content...