Organoselenium-catalyzed synthesis of indoles through intramolecular C–H amination†
Abstract
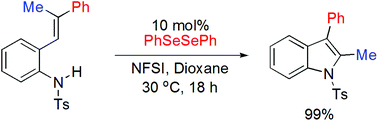
A new and efficient route for organoselenium-catalyzed synthesis of indoles via intramolecular C–H amination has been developed. The reaction conditions were mild and the desired products were formed in up to 99% yield. When the method was applied in the reaction of a trisubstituted alkene, the corresponding indole was obtained in 99% yield via 1,2-phenyl migration.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...