Syntheses of sceptrins and nakamuric acid and insights into the biosyntheses of pyrrole–imidazole dimers†
Abstract
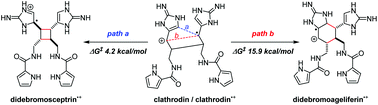
Sceptrins and nakamuric acid are structurally unique antibiotics isolated from marine sponges. Recent studies suggest that the biosynthesis of these dimeric pyrrole–imidazole alkaloids involves a single-electron transfer (SET)-promoted [2 + 2] cycloaddition to form their cyclobutane core skeletons. We describe herein the biomimetic syntheses of racemic sceptrin and nakamuric acid. We also report the asymmetric syntheses of sceptrin, bromosceptrin, and dibromosceptrin in their natural enantiomeric form. We further provide mechanistic insights into the pathway selectivity of the SET-promoted [2 + 2] and [4 + 2] cycloadditions that lead to the divergent formation of the sceptrin and ageliferin core skeletons. Both the [2 + 2] and [4 + 2] cycloadditions are stepwise reactions, with the [2 + 2] pathway kinetically and thermodynamically favored over the [4 + 2] pathway. For the [2 + 2] cycloaddition, the dimerization of pyrrole–imidazole monomers is rate-limiting, whereas for the [4 + 2] cycloaddition, the cyclization is the slowest step.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...